“Interesting metaphor. We’re perfectly imperfect designs.”
― Nina Riggs, The Bright Hour: A Memoir of Living and Dying
Cancer, which mirrors Virchow’s principle of Omnis-cellulae-e-cellulae (all cells come from cells), is a disease that arises out of the very same innate building blocks of the body it resides in. We think of cancer as a terrible foreign entity, a disease – but it is after all, the daughter of a cell that makes up the very body that harbors it. Formidable in its amorphous nature, cancers are terrifyingly complex in their evolution, the treatment of which is equally so – how do we target a cancer cell that harbors vast remnants of genetic material similar to the surrounding normal cells? Their camouflage, despite the “imperfections” or accumulated mutations from cellular divisions and aging, bring to the forefront a need to specifically target cancer cells…
…A need for tumor-informed approaches.
Through the course of history, science has precipitated nuances of understanding that have built up an arsenal of diagnostics, therapeutic interventions, monitoring methodologies that have culminated in an overall increased survival, better prognosis and improved quality of life compared to the previous century. Of note, the seminal shift from non-specific chemotherapy towards an era of targeted therapeutics and precision oncology underscores the potency of tumor-informed approaches wherein mutations that are exclusive to the tumor are identified and targeted. However, while individual battles are won – the victory of imatinib in BCR-ABL mutant chronic myeloid leukemia and vemurafenib in BRAF V600E mutant melanoma to name a few – the war still recurs.
History has a subtle way of infusing its essence through the course of time and human evolution
While there are several prominent moments of discovery that shine through in the mêlée between science and cancer, a hidden gem from the 1950s when Chinese-American oncologist Min Chiu Li treated his choriocarcinoma patients with anti-folate, is worth a mention.
The anti-folate treatment was considered a breakthrough at the time for choriocarcinoma patients – radiological scans showing complete ablation of gigantic tumors and distant lung metastases, a rare victory that propelled the field forward. However Li was a man possessed; he was intrigued by the human choriogonadotropin (HCG) secretions of the tumor and obsessively tracked the HCG levels in his patients’ blood. While the tumors shrank to oblivion on radiological scans, the HCG mirrored the regression of the cancer and plummeted, but not quite to zero – it persisted at low detectable levels. Li reasoned that while the HCG levels persisted, so did the cancer – undetectable on radiological scans, but quietly secreting their marker, leaving traces of their imprints in the patients’ blood. He did the unthinkable at the time, he continued to treat the patients with anti-folates, systemically subjecting them to additional rounds of toxicity while radiological evidence demanded him to stop, till the HCG finally plunged to zero.
The NCI was furious, and Li was fired, but several years later patterns emerged. While those who were treated solely based on radiological evidence invariably relapsed, Li’s patients who endured additional rounds of treatment based on their HCG levels stayed in prolonged remission – in essence Li’s extended treatment destroyed the remnants of their cancer that were undetectable on X-rays. It was a cathartic moment where science realized that …
…cures are only as effective as the technology that provided the readout.
Lessons from history provide a powerful impetus in the quest for battling cancer. From Li’s encounter, the radiological evidence, while still a gold standard in today’s treatment methodology was shown to be substandard to a blood test where the molecular imprints of cancer could be detected. And while the field has certainly evolved, we constantly strive to move the needle to improve outcomes.
Classical detection of molecular residual disease (MRD) after treatment is limited by conventional detection methodologies. Today, ctDNA Assays, where circulating tumor DNA in blood can be utilized to provide comprehensive insights on a tumor’s presence and its complex heterogeneity, have reimagined the possibilities for cancer detection, treatment, and monitoring. Harnessing a tumor-informed, whole genome approach, NeXT Personal™ draws on the ability to detect tumor variants in orders of magnitude higher than tumor-agnostic approaches or those that are limited to only tracking variants from the exome. We treat only that which we see – wouldn’t it be revolutionary if we could detect the smallest bit of cancer, as low as one mutant molecule among a million? NeXT Personal accomplishes just that – its limit of detection (LoD) is pioneering the next generation of MRD assays, with an unparalleled detection threshold at 1 part per million (1PPM), a 10-100x improvement over current technologies (Figure 1).

Figure 1: Representation of the LoD of the NeXT Personal assay – with an unparalleled detection threshold at 1 part per million (1PPM), a 10-100x improvement over current technologies.
“You can’t ever reach perfection, but you can believe in an asymptote toward which you are ceaselessly striving.”
― Paul Kalanithi, MD., When Breath Becomes Air
With NeXT Personal, we’re that much closer to perfection. The enhanced LoD is paired with the ability to track variants, and is a potent combination because now we not only have an MRD assay that detects recurrence in its nascent stages, but also sheds light onto the evolving mutational landscape of the relapsed cancer. Thus, malignancies can now be detected much earlier (often even prior to radiological presentation) and treatments can be swifter and informed, tailored to combat the emerging resistance variants. NeXT Personal is futuristic in its design, not only does it track mutational variants already present in the tumor, there is the added feature for surveillance of clinically relevant variants/emerging resistance mutations – this ability to examine not just the evolution of existing mutations, but also those that are likely to emerge as the tumor evolves over the course of treatment and time, provide formidable insights into tailoring therapeutic interventions (shown in Figure 2).
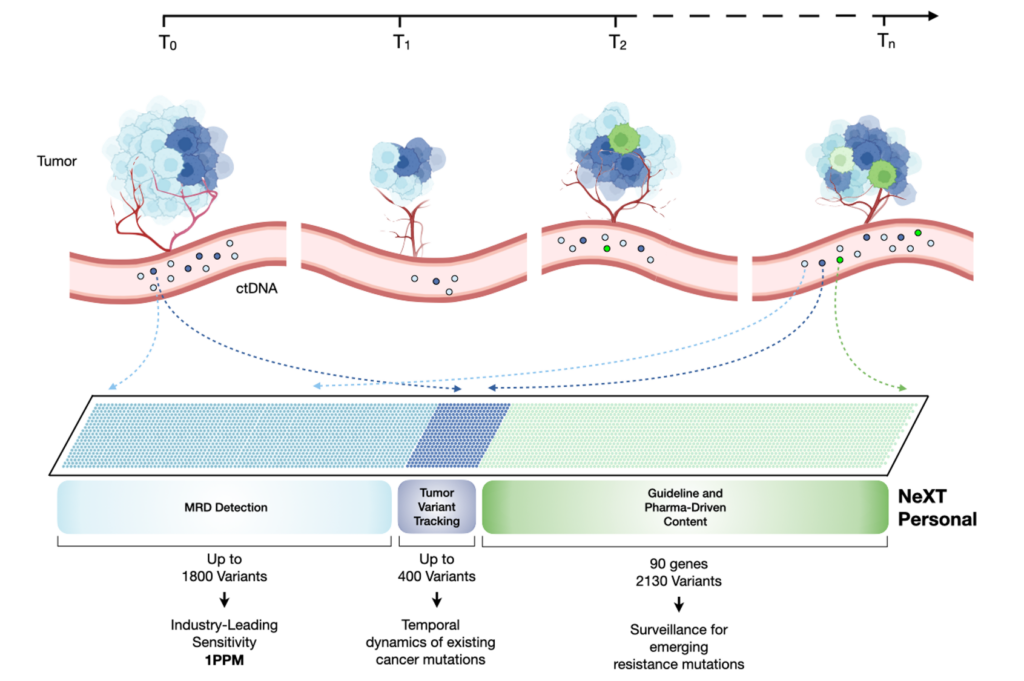
Figure 2: Overview of how the NeXT Personal assay interrogates existing and emerging mutations as the cancer evolves
This ability to inform treatment from ctDNA tracking of mutational variants was recently reported in the PADA-1 breast cancer trial. Mutations in Estrogen Receptor Alpha (ESR1) are known to confer resistance to first-line aromatase inhibitors in hormone receptor-positive, HER2+ metastatic breast cancer. Upon identification of the ESR1 mutation in plasma (even prior to disease progression), patients were switched from an aromatase inhibitor to fulvestrant, which doubled their progression-free survival, compared to patients who switched to fulvestrant after clinical progression. These findings underscore the relevance of early detection of tumor biomarkers that can inform therapeutic decisions.
“As soon as a patient lies down on the exam table, she has laid down her life on a bed of narrowed answers, but the questions are never sufficiently clear.”
― Anne Boyer, The Undying
At Personalis we do not question why this is important anymore, because we know. While innovation no doubt spears breakthroughs, innovation is in itself fueled by the most subliminal stirrings of the human mind: the central pillars of hope and striving. We stand on mountains of discoveries as we engineer our technological offerings; as several studies coalesce on the need for multidimensional biomarker analysis – be it combining insights from exome/transcriptome in the context of tumor-immune microenvironment or incorporating diverse metrics like HLA mutations and LOH while visualizing neoantigen burden and immune response, we provide powerful insights when combining the longitudinal monitoring capabilities of NeXT Personal with our comprehensive tumor tissue profiling platform ImmunoID NeXT®.
A question that motivates us is: what impact we can bring to patient lives and further cancer research? Our innovations are inspired, because our tumor-informed insights are not just at the NeXT™ level – they are Personal.
Contact us to learn more about NeXT Personal and NeXT Liquid Biopsy®.
All products described here are for Research Use Only and not for use in diagnostic procedures (except as specifically noted).
References
- Riggs, Nina (2017). The Bright Hour. Simon and Schuster
- Wright NA, Poulsom R. Omnis cellula e cellula revisited: cell biology as the foundation of pathology. J Pathol. 2012
- Siegel, RL, Miller, KD, Fuchs, HE, Jemal, A. Cancer statistics, 2022. CA Cancer J Clin. 2022
- Druker et al., Efficacy and Safety of a Specific Inhibitor of the BCR-ABL Tyrosine Kinase in Chronic Myeloid Leukemia. April 5, 2001 N Engl J Med
- Chapman et al., Improved Survival with Vemurafenib in Melanoma with BRAF V600E Mutation. June 30, 2011N Engl J Med
- Cancer of the placenta
- Li, Min C. (15 September 1979). “The historical background of successful chemotherapy for advanced gestational trophoblastic tumors”. American Journal of Obstetrics and Gynecology.
- Mukherjee, Siddhartha (2011). The Emperor of All Maladies: a biography of cancer
- National Cancer Institute
- Moding EJ et al., Detecting Liquid Remnants of Solid Tumors: Circulating Tumor DNA Minimal Residual Disease. Cancer Discov 1 December 2021
- NeXT Personal, https://www.personalis.com/next-personal/
- Paul, Kalanithi (2016). When breath becomes air
- Bidard FC et al: Fulvestrant-palbociclib vs continuing AI-palbociclib upon detection of circulating ESR1 mutation in HR+ HER2– metastatic breast cancer patients: Results of PADA-1, a UCBG-GINECO randomized phase 3 trial. 2021 San Antonio Breast Cancer Symposium.
- Boyer, Anne. The Undying: Pain, Vulnerability, Mortality, Medicine, Art, Time, Dreams, Data, Exhaustion, Cancer, and Care. Farrar, Straus & Giroux.
- Pich O et al., The translational challenges of precision oncology. Cancer Cell. 2022 May 9;40(5):458-478.
- Abbott et al., Prediction of Immunotherapy Response in Melanoma through Combined Modeling of Neoantigen Burden and Immune-Related Resistance Mechanisms. Clin Cancer Res. 2021 Aug 1